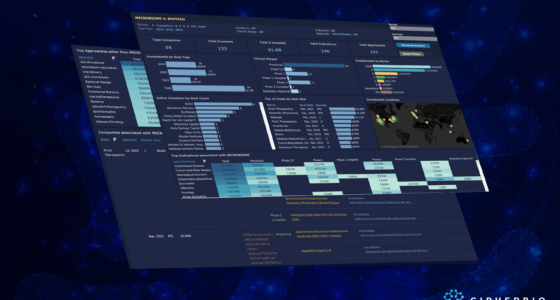
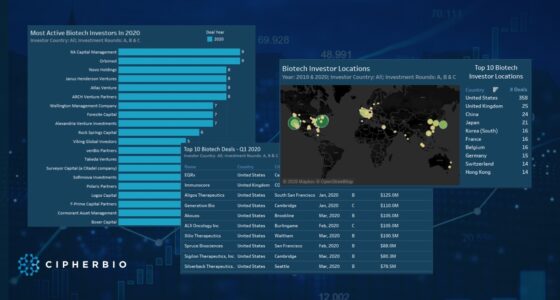
Until today’s date (October 2020), COVID-19 has killed over one million people worldwide, and still has not been contained. Cases are resurging in many places around the world, and scientists, laboratorians, and bioethicists are working around the clock to produce successful therapeutics, drugs, and a COVID-19 vaccine. Back in April, we looked at the early efforts by various actors in the field to put an end to the virus, and in this brief report we look at the progress that has been achieved since then.
As life science companies and institutions learn more about the virus, they are becoming better at identifying what works and what does not, and with which patient segments. According to one estimate, there have been over 3500 studies around the coronavirus as of early October this year. The bulk of those studies are being conducted in Europe and North America. Currently, many companies are running trials to identify effective and safe drugs, and there are more than 150 COVID-19 vaccines under development around the world. The life science sector has become faster at responding since previous pandemics with deeper knowledge and better technology, but the race against time continues.
Drugs and Therapeutics Against COVID-19
Many drug trials are currently being conducted to discover, develop, and produce a drug that helps COVID-19 patients.
Earlier this year, the FDA gave emergency use authorization (EUA) to Remdesivier, a drug that is administered intravenously and developed by Gilead Sciences.
COVID-19 Convalescent plasma (CCP). The FDA has also granted an emergency use authorization to treatment by convalescent plasma, which is donated by patients who recovered from COVID-19. Although small studies have shown that this treatment increases survival rate, there needs to be wider studies to confirm its effectiveness.
Some anti-inflammatory drugs have been found to work on severe cases but not milder ones. Other drugs, such as favipiravir and merimepodis are being tested. Generally, the drugs being tested fall into one of three categories: Antiviral drugs that target the virus and its functions, drugs that calm down the immune system, and drugs that deliver antibodies from patients who recovered to current patients.
While we do not yet have a definite therapy for patients with COVID 19, many treatments are being tested to find out if they carry any promise. Immune response modulation and inflammation management are essential in any potential treatment.
Aclaris Therapeutics is currently conducting clinical trials of its drug ATI-450 on mild and severe cases of COVID 19. The drug is used for the treatment of rheumatoid arthritis and now it is being investigated for its efficacy and safety for patients with COVID 19. The drug, called ATI-450, is an oral small molecule MAPKAPK2 (MK2) inhibitor that potently inhibits multiple inflammatory cytokines. Cytokine Release Syndrome (CRS) has been associated with mortality among COVID-19 patients.
Olumiant (manufactured by Eli Lilly, a pharmaceutical company) is also another drug that is normally used to help patients with rheumatoid arthritis, and it is being tested to see whether it can help COVID-19 patients as well. When Oluminat was coupled with Remidisivir, the two drugs were found to produce benefits among patients in early studies. The treatment reduced recovery time by 12.5% and improved chances of improvement by 30% after 15 days.
Dexamethasone went into clinical trials in June, in which patients received low doses of the drug. Early results indicated that the drug reduced deaths by one third in ventilated patients, and by one fifth in patients who needed only oxygen. The drug showed no benefits to patients who did not need respiratory support.
Other drugs such as Aviptadil which has been granted Expanded Access Protocol by the FDA (the drug was originally developed by mondoBIOTECH and its patent is currently owned by Relief Therapeutics Holdings), and Tradipitant which is currently being studied (the drug was originally owned by Eli Lilly and now it is owned by Vanda Pharmaceuticals) also seem to be promising in their effectiveness with some COVID-19 cases.
Progress on a vaccine against COVID-19
Many companies have made progress when it comes to the vaccine despite some hurdles. Below, the ones who are closest to a successful vaccine are highlighted.

Moderna, is a biotech company that was co-founded in 2010 by Robert Langer (a distinguished MIT professor) and Noubar Afeyan (the founder of Flagship Pioneering, a fund that has been a key backer of Moderna). It has been backed by many investors including Viking Global Investors, Abu Dhabi Investment Authority, and EDBI, among others.
Moderna began its vaccine clinical trials for mRNA-1273 (a novel lipid nanoparticle encapsulated mRNA-based vaccine) in March. It then expanded its patient base to include older adults to ensure that the results it is offering are reliable for more people, especially more vulnerable ones. At the end of September, Moderna confirmed that its vaccine produces an immune response in older adults (over 56 years old) and is well-tolerated by the people who received it. Scientists said that the blood of vaccinated volunteers contained robust binding and neutralizing antibodies against the coronavirus SARS-CoV-2.
Moderna’s CEO commented that the company does not expect to apply for emergency use authorization (EUA) before late November. The company wants to have gathered safety data from at least half the participants in its trials who have received their second doses. Thus far, the company seems on track to submit the application on time.
Vir Biotechnology in cooperation with GlaxoSmithKline PLC started the second trial (of three) at the beginning of September to test the effectiveness of its antibody treatment, which is called VIR-7831. The two companies announced the beginning of the second trial at the end of last August. If successful, the antibody would prevent hospitalization of COVID-19 patients, especially high-risk ones. The results may be available by the end of this year. However, access to the antibody may not be available before the second half of next year (2021).
The two companies announced that they have moved forward to phase 3, following positive evaluation of safety and tolerability data from phase 2 lead- in.
Vir Biotechnology is a biotechnology company based on San Francisco. It was established in 2016 to tackle infectious disease. It has received backing from investors such as Temasek, Arch Venture Partners, and Alaska Permanent Fund, among others.

Sorrento Therapeutics has currently many programs to tackle the COVID-19 pandemic, such as a diagnostic test, an antibody test, a neutralizing antibody cocktail, and a potential vaccine, among other programs.
In September, the company received approval from the FDA to proceed with trials for STI-1499, a neutralizing antibody. Investors welcomed the news and the company’s share price soared. Sorrento’s objective is to approach the FDA with an emergency use authorization application before the end of this year.
Pfizer has also been working on a potential COVID-19 vaccine in collaboration with BioNtech. The two companies had announced that they would expend their third clinical trial to include more participants as well as increase the diversity of the population. The study would include 44,000 participants, up from 30,000. Pfizer confirmed its commitment to high efficacy and safety standards before moving forward with the FDA approval.
The Janssen Pharmaceutical companies of Johnson & Johnson have begun phase 3 clinical trial of its investigative vaccine against COVID-19 (called JNJ-78436725 or Ad.26.COV2.S), however, it has been temporarily paused and is currently pending review. The aim of the test is to discover whether the vaccine will prevent the onset of COVID-19 and its symptoms. The Janssen vaccine candidate is a recombinant vector vaccine that uses a human adenovirus to express the SARS-CoV-2 spike protein in cells.
The trial is funded by Janssen, the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, the Biomedical Advanced Research and Development Authority (BARDA), and part of the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response.
The Published preclinical findings show that the vaccine induced an antibody response and provided complete or near complete protection of the lungs and the nose. Safety evaluations have not been finalized yet, but they warrant further investigation of the vaccine candidate.

Novavax, a company specialized in manufacturing vaccines against infectious disease, is still working on its NVX‑CoV2373 vaccine candidate against COVID-19. The preclinical data showed that the candidate induced antibody response which helps to block binding of the virus with cells. Phase 1 trial showed that the vaccine candidate was well-tolerated by the patients who received it, and that the immunity response was good. Phase 2 trials were initiated in August.
Inovio Pharmaceuticals concluded the phase 1 clinical trial of its vaccine against COVID-19, and the participants in the trials showed improved immunological responses. The phase 2 clinical trial began in September. Inovio expanded its manufacturing capacity to be able to make 100 million doses as of half 2021 in cooperation with its numerous partners. Inovio Pharmaceuticals is a biopharmaceutical company with focus on DNA products to treat cancer among other diseases.

The Murdoch Children’s Research Institute is trying to test whether the Bacillus Calmette-Guerin (BCG) vaccine can help against COVID-19 disease. The vaccine has been used to prevent tuberculosis for nearly a decade. Given its benefits to the immune system, scientists are investigating whether it can also help coronavirus patients or vulnerable groups. Randomized trials of the vaccine began in April, with the aim of finding evidence that the BCG vaccine can protect against the novel coronavirus SARS-CoV-2.
CanSinoBio and the Academy of Military Medical Sciences are working on a vaccine that elicits a response, which can be either a T cell response or antibodies. Many trials are currently being conducted in various locations around the world, and they have not been finalized yet. CanSinoBio is a biotech company that has been backed by investors such as Lilly Asia Ventures, and SDIC Fund Management, among others.
Register for a free CipherBio account to find exclusive life science industry insights.
Diagnostic Tests
Although the coronavirus is fairly novel, there are now already more than 350 diagnostic and testing products approved and available on the market, with the potential of having more in the near future. Some of those tests are better than others. Better testing means faster detection of positive cases, including asymptomatic ones, which in turn means better tracking of infection resulting in better containment of the virus.
Test Highlights
Roche has received an emergency use authorization from the FDA for its antibody test, called Elecsys®. The test detects antibodies in human serum and plasma. The company has developed other tests such as real time PT-PCR test, and real time PT-PCR mPOC test.
Abbott has developed a test that is rapid to detect COVID-19 called BinaxNOW™, and is paired with a mobile app. The company plans to produce 50 million tests in October this year.
Cepheid has developed a test to detect the coronavirus that causes COVID-19. The test is fast as it can detect the virus within 30 minutes.
Hologic has received emergency use authorization from the FDA for its molecular test called Panther Fusion® SARS-CoV-2 test, which can detect the virus by detecting its genetic material, in less than 72 minutes.
Researchers led by Jeniffer Doudna (who was one of the Nobel Prize winners in Chemistry this year) have reported that they were able to make a diagnostic test that could detect the coronavirus in under 5 minutes based on CRISPR gene-editing technology. The test is remarkable in its speed and appears to be reliable. It has been developed by the University of California San Francisco and Mammoth Biosciences. Jennifer Doudna is a cofounder of Mammoth Biosciences, and the chair of the scientific advisory board. Mammoth Biosciences is a diagnostic company with focus on CRISPR gene-editing technology, and it has been backed by investors such as aMoon Fund, Mayfield Fund, NFX, and others.
CureBase has launched a platform called cure-19 to help run clinical trials for COVID-19 at home, which helps reduce infections and makes trials run faster. Y combinator and C3 Ventures are among the backers of CureBase.
Other companies, like ContinUse Biometrics (which makes contact-free sensing platform which can detect symptoms such as those associated with COVID-19), PathGroup (which provides laboratory equipment and received a grant from NIH to expand its COVID-19 testing laboratory), Luminostics (which joined forces with Sanofi to develop a COVID-19 self-testing solution) are also contributing in their own ways based on their cope capabilities.



Challenges facing the life science sector in its endeavor to combat COVID-19

Despite the rush to come up with effective drugs and a vaccine, biotech companies still need to follow proper testing and safety guidelines . Various clinical trials have resulted in some intense side effects among recipients of the potential COVID-19 vaccines. The trials continued after ensuring it was safe to resume.
The FDA is aware of the need for high safety standards. It is currently planning to have any application for emergency use authorization reviewed by an expert panel, in addition to requiring at least two months of safety data. Companies and institutions seem to be giving proper emphasis to both efficacy and safety, and understand the importance of both.
Even if effectiveness and safety data is provided, there remain numerous challenges, which are mostly related to manufacturing and logistics. To deliver a vaccine to a global population of over 7 billion, large production and distribution capacity is needed. This practically cannot succeed without the cooperation of various actors from various countries.
We are learning more every day about the coronavirus, its impact on human health, and the people who are most vulnerable. We may not have a vaccine yet, but we are getting closer to one every single day. The life science sector won’t relent until we reach one.
Join us in our effort to capture the entire life science ecosystem in our database.
We strongly believe that the availability of a high-quality database about the life sciences ecosystem increases sector efficiency and benefits every company raising capital. Thus, if you are an investor or a startup in any of the relevant industries, we would like to extend an invitation to you to join the platform and or subscribe to the CipherBio insight newsletter.
Fill out your digital profile, or update it with your company’s latest news, so that you can be a part of the data set that represents the life science ecosystem.